Cave Fauna of Washington Caves.
By Rod Crawford written mostly 1984.
Cave ecology classifies animal species into four basic categories based on how dependent they are on subterranean environments Troglobites are those species restricted to subterranean niches (caves and smaller bedrock cavities) and unable to survive on the surface, even in soil and litter (aquatic troglobites are often called stygobionts)
Troglobites — and their aquatic counterparts, sty- gobionts — tend to capture the most attention in cave biology because these species are unique to the subterranean environment, and can never be seen elsewhere The loss of eyes and pigment, elongation of appendages, etc, in many of these species also arouses interest It is seldom possible to encounter such life other than in caves, and this led most earlier workers to imagine that the major populations of troglobites were in caves penetrable to man It is now known that troglobite habitat is much more extensive than this, and includes what Howarth calls “mesocaverns,” cavities ranging from 1 mm to 20 cm wide Such cavities are often widespread throughout a given rock formation, thus making it permeable both to groundwater and to subterranean life.
Groundwater permeability can be measured, and is found to reach its maximum in gravel, limestone, and basalt There are no caves in gravel, so caves in limestone and basalt are where subterranean life is most accessible to man; such caves are our “windows” into the subterranean world, and their protection is thus crucial if we are to have continued access to this world The troglobites of limestone caves have been well known for years, but the investigation of troglobites in basalt only began seriously in the 1970s, and this fauna is still very poorly known Intensive studies have been done in two areas of Japan, in the Galapagos, and in Hawaii (where the most diverse fauna, with about 50 troglobite species known, was found), and by Senger and Crawford at Mt St Helens.
Since individual lava flows may be rather short- lived on an evolutionary time scale, the most diverse basaltic subterranean faunas tend to develop in areas with many adjacent flows, where fauna can migrate from one flow to another. Except for Mt. St. Helens, the areas previously studied (see above) are like that Such is also true of the Mt Adams area of Washington, where a little random collecting in about a 20- year period produced at least 15 subterranean Species, doubtless only a fraction of the total These include a flatworm, an isopod, an amphipod, a copepod, three rhagidiid mites, a water mite, a harvestman, a pseudo- scorpion, four millipeds, and a campodeid. Only six of these have been formally described as yet. Three of the aquatic species and seven of the terrestrial species are large enough to be easily recognized by the average cave visitor.

elliotta mite by Crawford
In the caves of the Mt St Helens Cave Basalt, 10 terrestrial troglobites (or possible troglobites) have been found. Most are tiny; only a campodeid bristle- tail, and a rare pseudoscorpion, are large enough to be noticed by the average caver Four aquatic stygobi- ont species were found, all in Little Red River Cave, though one (and most probably the others) also occur in the Falls Creek System to the east Of these aquatic species, only an amphipod and a flatworm are large enough for the average caver to notice.

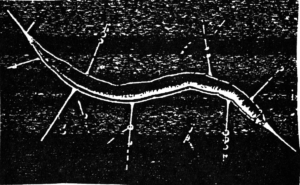
Stygobromus quatsinensis, stygobiont amphipod from
Vancouver Island (about 7 mm long). Washington species is similar.
Species in the second ecological category, troglophiles, have permanent resident populations in both cave and surface situations, with numerous species in the dark zone, many others being residents only in cave twilight Flies dominate this category numerical- ly Also important among the detritus feeders are Col- lembola (the tiny “springtail” insects), the millipeds, and various mites Some sapsucking insects are found on roots that penetrate the cave ceilings Troglophilic predators include spiders, harvestmen, grylloblattids, and beetles Several spider species are common in en- trance and twilight niches, feeding on numerous trogloxene (see below) flies that enter caves for shelter.

Springtail, tomocerus Californicus
The trogloxenes are species regularly found in caves but not spending their entire life histories under- ground A few, most notably the camel crickets, rest in caves by day and emerge at night to forage, as do bats among the vertebrates Numerous nematocerous (mosquito-shaped) flies and some other insects use cave entrances casually for resting or swarming Other species habitually overwinter in caves, such as the Geometrid moth Triphosa haesitata, the Noctuid moth Scoliopteryx libatrix, and the harvestman Nelima paessleri.
“Accidentals“, the last major cave ecological cat- egory, are species which wander into caves but can- not survive there Perhaps one-third of the species that may be collected in caves are accidental Minor categories include parasites and phoretics (hitchhikers) on trogloxenes (such as mammals or millipeds), which, though interesting, have little relevance to the cave communities.
COMMUNITY ECOLOGY
Energy Sources
Since there can be no green plants in absolute darkness, and thus no direct conversion of sunlight into living matter by photosynthesis, cave ecosystems depend for their existence on organic matter brought in from outside A usually unimportant exception is autotrophic bacteria that gain energy from inorganic chemical reactions; the role of such bacteria in shallow caves like lava tubes is insignificant. Organic matter is carried to cave passages (and surrounding smaller cavities) directly from the surface through the overburden via seepage water, roots, and very small burrowing invertebrates It is brought into caves through entrances via gravity, flowing water, vertebrates and invertebrates that enter caves accidentally or as part of their life history, and man These energy sources are the entire foundation for the cave food chain; without them, cave life could not exist, and any change in them will be reflected in the cave ecosystem. In the following paragraphs, various energy sources are discussed as they relate to Washington lava tubes, with some indication(highly speculative) of the cave fauna that make use of them there.
Slime nutrients Water continually seeps into most Washington cave passages from the surface It carries with it organic matter as dissolved chemicals, colloids, and fine particles. When this material reaches cave walls (as well as cracks and other cavities), it supports the growth of “lava tube slime” A study of slime in Slime Cave and New Cave (Staley and Crawford 1975, Cascade Caver 14: 20-21) showed that it consists of heterotrophic bacteria that live on organic matter already formed in and by other organisms) and their dead remains.
Of the varied bacterial species present, actinomycetes were
dominant Actinomycetes are bacteria that share some properties of fungi, such as production of filaments and spores They are slow-growing organisms that specialize in breaking down more resistant organic molecules than those favored by faster-growing bacteria and fungi; they favor a neutral or alkaline pH and are not tolerant of acid conditions Individual bacteria numbered 29-42 million per gram dry weight of slime Their reproduction and growth is extremely slow, so that damaged slime will take a long time to grow back At temperatures of about 6° C or higher, the slime organisms develop spores and associated structures, which form a thin, white or silvery coating on the surface This material is hydrophobic and causes water drops to bead up It is the “white slime” seen in warmer parts of caves At lower temperatures (or other stressful conditions), the same organisms produce no spores, revealing the brown-orange material which underlies the white coating elsewhere; this is “orange slime.”
Slime is thus one of the most abundant food resources of the cave communities However, the only common animals known to feed on it directly are the translucent, web-weaving fungus gnat larvae, Spe- olepta spp These occur on walls and ceilings in all caves but those having little slime We might speculate that anoetid mites and other microscopic invertebrates also have an important, but less visible, role in slime consumption.
Speolepta larva, length 12mm.
Roots. Roots of trees and probably other plants grow down through cracks in the lava and penetrate the ceilings of Washington lava tubes, primarily in shallower passages with less than 15 feet of overburden Roots from Cougar Cave (Mt St Helens) were tentatively identified as being from lodgepole pine, and probably those in most of the caves are from coniferous trees Roots are certainly of some importance to these caves, but in Hawaii their role is a dominantone (discussed in various papers by Howarth) The Hawaiian tree Metrosideros collina sends roots 30 feet or more through the young lava, often nearly blocking cave passages Feeding on these Hawaiian roots are five troglobitic species, while other species scavenge dead roots on the floors.
The two invertebrate species definitely linked with root feeding at Mt St Helens were found on roots in only one cave each: the cixiid planthopper Cixius lo- chites, associated with roots in Beaver Cave, and the ortheziid root scale insect Arctorthezia occientalis, found only in Beaver Bay Cave At Mt Adams, the troglobitic milliped Lophomus skamania has been photographed feeding on roots The larvae of sciarid gnats, found in most of the caves, might feed on roots, where they could easily escape notice White fungi grow on the roots in many of the caves.
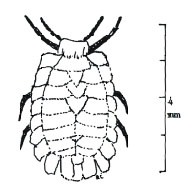
Root Scale insect, Arctorthezia occidentalis
Organic detritus Flood-washed debris is the major organic input to many limestone caves, but is comparatively rare in lava tubes Deadhorse Cave (Mt Adams) is the major exception in this area, where the cave stream originates partly from groundwater and partly from nearby Deadhorse Creek, carrying much organic matter, the bulk of which seems to be wood particles.
At least some debris, ranging from logs to fir needles, falls into entrances of most caves Skylight entrances receive the brunt of this This material does not ordinarily penetrate beyond twilight, and the animals living on it are threshold troglophile species of Collembola, beetles, flies, ants, mites, and worms. Some dark zone troglophiles overlap with this threshold debris community.

Epigean vertebrates On numerous past and recent occasions, “accidental” vertebrates (such as rabbits) have wandered into these caves and died there. The numerous skeletons found support this. “Accidental” amphibians, often found in the caves, particularly Ole’s Cave, do have resident salamanders. Also, finding of bodies of skeletons proves that trogloxenic animals that use the cave regularly, such as bats, mice, and pikas, die there on occasion. The carcasses of such animals provide a “windfall” of food for the cave community when present. The first to exploit it are apparently the trichocerid gnats. Chironomid gnats may join the feast at a later stage. When most of the flesh is gone, fungi grow on the fur and bones, supporting other organisms.
Trogloxenic mammals (part-time cave residents) contribute a variety of food resources for cave life, though mostly in twilight. Woodrat nests have a self- contained animal community when active, but after abandoned join the general reservoir of organic detritus. Haystacks and stick piles doubtless grow mold which supports various species. Scats of mice, woodrats, and pikas occur well into the dark zone, where the mold they grow supports Collembola, millipeds, mites, and others. Deer mice and woodrats apparently scat- ter their parasites and other nest fauna here and there throughout most of the caves, where many of these “lost” parasites doubtless fall prey to cave predators. Substantial amounts of bat guano are rare in Washington caves but a little can be found occasionally. The midden deposits of moth wings found under bat night roosts are probably more important. They undoubtedly grow fungi which support mites and Collembola, and probably other life; it is evident that these deposits, formed mainly in late summer, are largely consumed in the course of a year, since only traces are found earlier in the summer
Epigean invertebrates. Numerous gnats and other flying insects that shelter in cave entrance and twilight areas are drawn by air currents in the dark zone, there to perish,as shown by collections from drip pool sand pitfall traps. Many of these are eaten by cave predators and scavengers Thosethatfallinthewa-teri Little Red River Cave(and other caves with permanent water)maybe eaten by flatworms.In Twilight Areas, they are the mainstay of the twilight spider species Slugs and snails often wander far into caves and cannot survive there,and these provide a more substantial food resource The invertebrates that hibernate in caves are doubtless often eaten by cave predators, perhaps mainly bats and mice in case of the moth, but the smaller species also by grylloblattid, crickets, beetles, or harvestmen.
Human deposits . Wood, deposited by man, is to be found in several of the caves. This wood supports fungi and bacteria which in turn supports other life. Leavings of human food are to be found in the most popular caves. In Ape Cave (Mt. St. Helens), this resource has resulted in a permanent “population boom” of trichocerid gnats, and has allowed the spread of threshold species such as the sciarid gnat Lycoriella mali throughout the cave. Predators such as campode- ids and grylloblattids sometimes come to this resource, but other human-associated impacts to Ape Cave have apparently caused them to be more cryptic in their habits, and they have much lower populations than the numbers of gnat prey could potentially support there.
Dark Zone Community
To summarize the preceding section: the most important energy sources for dark zone communities in this area are slime, roots, carcasses and scats of epige- an vertebrates, accidental invertebrates, organic detritus in groundwater, and, in some caves, human deposits. Speolepta larvae are the most important primary consumers of slime known throughout the area. Roots are consumed by sucking insects and millipeds. Vertebrate carcasses are consumed by fly larvae (Tricho- cera and Hydrobaenus). Nothing was found feeding directly on bat guano or other mammal scats, but such fauna may exist. Accidental invertebrates probably provide a significant food resource for many cave predator/scavengers, which in such cases are primary rather than secondary consumers. In caves with permanent streams, the organic content of groundwater and pirated surface-stream water supports stygobiont crustaceans indirectly via bacteria etc., perhaps also directly human deposits in popullar caves seem to support mainly fungi, flies, and their predators.
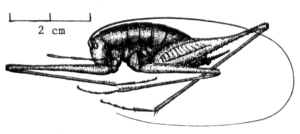
Giant camel cricket Tropidischia xanthistoma, “folded up” to fit
Roots, older carcasses, detritus of many kinds, dead invertebrates, and particularly mammal scats, support various molds and other fungi These in turn are eaten by Collembola(tiny”springtail”insects), flies, millipeds, and presumably some mites. Root fungi are eaten by plant hoppers and probably others.
Important smaller predators in the dark zone include campodeids, Haplocampa; harvestmen, Spelonychia sengeri (Mt. Adams caves only); mites, Elliotta; and sometimes the uncommon trogophile spider Nesticus silvestrii Medium-sized dark zone predators include the smaller crickets, Pristoceuthophilus spp. ; beetles; and, in groundwater, the flatworms. The largest invertebrate predators are the grylloblattids.
The top predators in most of the caves (at Mt. St. Helens) are presumably deer mice, which certainly eat grylloblattids and staphylinid beetles, as shown by scat contents, and probably eat the other large cave invertebrates as well. They, in turn, have many predators outside the caves.
Threshold Communities
Troglophile milliped, Kepolydesmus mimus
In the cave threshold communities of this area,
there is little slime (but occasional occurrence of Speo- lepta), and while roots may be present, the root feeding species have not been found Vertebrate carcasses seem to have the same sort of fauna as in the dark zone, with the probable addition of phorid flies (Megaselia inornata) Debris washed or fallen in, old woodrat nests, bat middens, etc, are present much more abundantly than in the dark zone, and support a richer and denser community of detritus feeders, including gnats (Trichocera, sciarid and chironomid), phorid flies,beetles (Catops), ants (Lasius),and worms (Mesenchytraeus)Fungi growing on such material likewise support numerous Collembola, sciarid gnats, millipeds (Kepolydesmus) , and mites.
Some of the dark zone predators venture into twilight occasionally or regularly, including the campodeids, crickets, grylloblattids, carabid staphylinid beetles, harvestmen, some mites, and of course mice. The threshold community also has its own predators such as certain beetles and mites, spiders (Bathyphantes, Calymmaria, Metellina, and particularly Pimoa), salamanders, and non-hibernating bats. These doubtless prey on resident species, but their mainstay is probably the numerous insects and other invertebrates which enter the cave threshold for shelter or hibernation Many cave biologists disdain the threshold threshold community and its fauna as not worth studying It is, however, a distinct assemblage of species different from either the dark zone or the outside world, and I find it very interesting in its own right.

Grylloblatta chirurgica in Little Red River by Matthew Farnell

Grylloblattids
CAVE FAUNA OF OREGON CAVES
The Oregon High Desert Grotto has a excellent report on their website on cave fauna. Click here

Salamander
